- Completion and upload to LONI the Methods Report, ADNI3: Batch analyses of Ab42, t-tau, and p-tau181 in ADNI1, GO, 2 CSF samples using the fully automated Roche Elecsys and cobas e immunoassay analyzer system. This dataset, uploaded April 2017, includes a total of 2,401 never-before-thawed aliquots of ADNI1, GO and 2 CSF samples that had been collected between 9/7/2005 and 7/25/2016. See PPT set #101 for a description of major parts of the method validation for Ab42, and some cut-point estimations and see PPT set # 102 for further analyses, determinations of cut-points and relationships of abnormal and normal biomarker results to cognitive decline and progression from MCI to AD dementia done so far and working toward:
- definition of cut-points for Ab1-42, t-tau, p-tau181 and the ratios, Ab1-42/t-tau and Ab1-42/p-tau181 using Mixture Modeling and ROC analyses;
- an understanding of the predictive performance for cognitive decline and progression of MCI participants to a clinical diagnosis of AD dementia using these cut-points;
- Assessments of the comparisons between Ab-|tau-, Ab-|tau+, Ab+|tau-, and Ab+|tau+ for predictive performance of each pair [Ab+ is below CSF cut-point value; Ab- is at or above CSF cut-point value; tau- is below and tau+ is at or above the cut-point value for CSF tau; analogous pairs for Ab and p-tau181] for cognitive, memory and functional decline and progression from MCI to a clinical diagnosis of AD.
-
- assessments of concordance between CSF Ab1-42, t-tau, p-tau181, the ratios, Ab1-42/t-tau and Ab1-42/p-tau181 and Florbetapir PET imaging-based plaque burden assessments.
- The inclusion of validated CSF Ab40 to Ab1-42, t-tau, and p-tau181 in ADNI3 will permit evaluation of the Ab1-42/Ab40 ratio for possible improvement over Ab1-42 alone for clinical utility.
A manuscript describing the concordance performance of ADNIGO/2 Roche Elecsys CSF Ab42, t-tau and p-tau181 biomarker data and that from the Swedish BioFINDER study with either Florbetapir PET or Flutemetamol amyloid PET imaging, respectively, in the respective study cohorts, has been accepted for publication (Hansson et al, 2018b).
Provided support for the development of new immunoassays for CSF Ab42, t-tau, p-tau181 by providing residual CSF aliquot samples to 3 vendor laboratories.
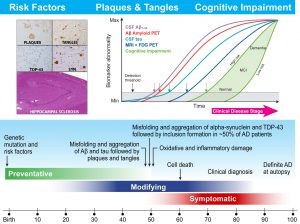
Figure 2. Timing for the onset and progression of AD
The figure below illustrates the timing for the onset and progression of the AD in the upper right panel with examples of mixed pathologies found in AD brains in the upper left panel while the lower panel summarizes the timing of pathology deposition and neuron death as well as current considerations for the treatment of AD. This figure is from a recently published update of earlier ADNI reviews from Kang et al, 2015, that provides our current understanding of the hypothetical timeline for the onset and progression of Alzheimer’s Disease neurodegeneration and cognitive impairments progressing from normal to mild cognitive impairment and then to Alzheimer’s disease dementia.