Leslie M Shaw and John Q Trojanowski
The Biomarker Core continues to receive, aliquot, store and curate all biofluid samples (CSF, plasma, serum) collected from subjects enrolled in ADNI3, including all who “carry over” from ADNIGO/2 and all newly enrolled individuals, with 24/7 surveillance in the ADNI freezers (-80 0C) that are housed in secure, dedicated space at UPENN. The updated (as of June 4, 2018) list of pristine aliquots of CSF, plasma and serum samples collected from ADNI subjects, can be found on the ADNI LONI website under BIOSPECIMENS, but below in Table 1 is a brief summary of these samples.
Table 1. Summary of ADNI CSF, plasma and serum samples received and aliquots prepared as of 7/18/2018.
| CSF | Ser + Pla | TOTAL | |
|---|---|---|---|
| ADNI 1 Primary Biofluids Collected | 1118 | 9756 | 10874 |
| ADNI 1 Aliquots in Bank | 24934 | 132581 | 157515 |
| ADNI GO/2 Primary Biofluids Collected | 1318 | 7816 | 9134 |
| ADNI GO/ 2 Aliquots in Bank | 35315 | 114919 | 150234 |
| ADNI 3 Primary Biofluids Collected – Rollover Participants | 118 | 667 | 785 |
| ADNI 3 Primary Biofluids Collected – New Enrollees | 162 | 402 | 568 |
| NOTE: Aliquots of ADNI3 fluid samples are in preparation & #’s will be available in next update of this table. Detailed aliquots report is available in Biospecimen section of “Access Data and Samples” drop down menu. | |||
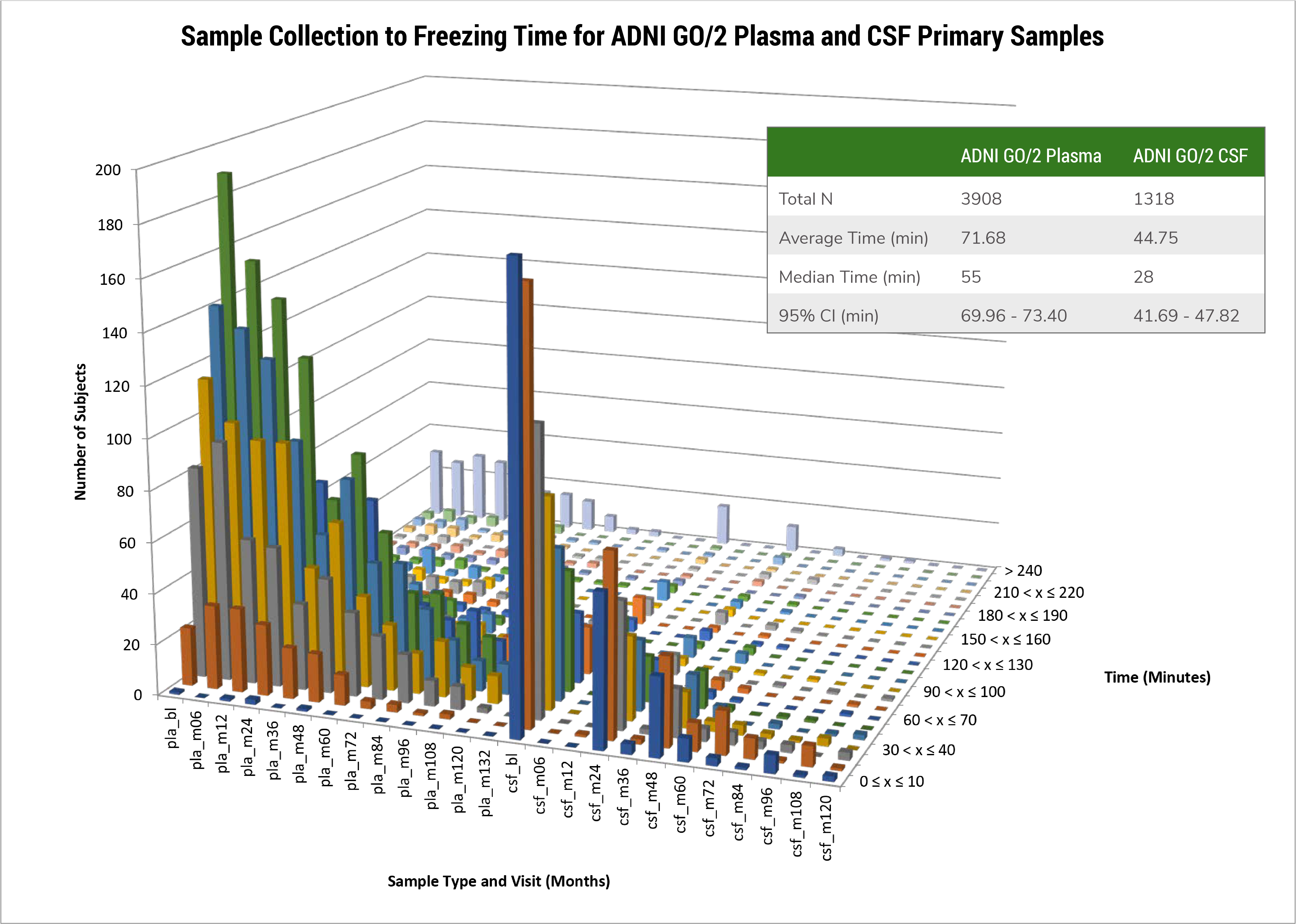
Biomarker Core staff continue to monitor details involved in the preparation of all Biofluid samples at study sites including time from obtaining each sample to the time of freezing on dry ice (a summary of this is provided in Figure 1 below). We continue to review details involved in the pre-analytical steps involved in biofluid sample collections. In the figure below a focus on sample preparation time shows that for CSF the mean, 95%CI and median values across 1,318 samples collected from ADNIGO/2 phase participants is: 44.8 min, (41.7-47.8 min) and 28 min respectively. For 3,908 plasma samples the respective values are: 71.7 min, (70.0-73.4 min) and 55 min. This information is available for each ADNI sample. The handling at each ADNI site of these biofluid samples is very important to assure the quality of each sample. Avoidance of hemolysis and time-efficient sample preparation are essential to the goal of sample quality. For plasma, the recommended time from collection to freezing is no longer than 120 min; for CSF the recommended time is 60 min or less in order to minimize risk for biomarker degradation due to various metabolic processes.
Figure 1. Sample collection to freezing time for ADNI GO/2 plasma and CSF primary samples
Biomarker Core staff regularly communicate with individual sites and Clinical Core staff regarding biofluid collections and any issues concerning sample quality, labeling discrepancies, and provision of updated samples-received summaries.
We are continuing our collaborative studies on identifying and controlling pre-analytical factors that can contribute to variability in CSF or plasma biomarker measurements especially A?1-42 (Vanderstichele, 2011; OBryant, 2015; Hansson, 2018). An example of this is a world-wide collaborative effort under the auspices of the Alz Association Global Biomarker Standardization Consortium(GBSC) whose members from industry and academic centers are defining a unified protocol for CSF sample collection for use in routine clinical practice that does not require freezing of CSF and another for large multicenter studies such as ADNI, wherein freezing bio-fluid samples prepared at 59 study sites and shipment to the Biomarker core laboratory is essential for assurance of sample stability for the long-term. The latter is also consistent with international treatment trials wherein long-term storage is an essential aspect for biomarker studies in bio-fluids. A new effort is the Biomarkers Consortium NSC Plasma A? Working Group that was recently organized to pursue various aspects of plasma A? measurement. Although there have been mixed results for plasma A?42/A?40 for accurate detection of AD using a number of immunoassay approaches, it is fervently hoped that by identifying and controlling pre-analytical factors, improved analytical techniques, and controlling for concomitant disease factors(Rissman, 2012) that progress can be made on improving the diagnostic utility of these measurements (Ovod, 2017; Nakamura, 2018; Song, 2018). We will provide updates of these developments at the annual ADNI Steering Committee meetings and FTF meetings with our ADNI PPSB colleagues.
RARC-approved studies using ADNI Biofluids
Biomarker Core staff prepare and ship bio-fluid samples(CSF, plasma or serum) to all investigators whose biomarker study proposals have been approved by the RARC (Resource Allocation Review Committee, appointed by the NIA) and following final review by NIA (see Table summarizing ADNI Biofluid Samples Sent to Investigators for RARC-Approved Studies for an up to date brief summary of these studies and status of results upload). Once completed the data from these studies, performed blinded, are uploaded on the LONI/ADNI website together with a Methods document that describes the methodology involved and quality control performance. Biomarker Core faculty, Drs Trojanowski and Shaw are happy to provide input on any study although this is not required but often we are asked. The procedure for making an application to the Resource Allocation Review Board(RARC) for ADNI biofluid aliquot samples is available on the Access Sample page.
A study that builds upon the 2013-2014 proteomic study that used LC/MSMS mrm mass spectrometry methodology will be conducted in 2018 to determine accurate concentration values for 5 candidate biomarkers that showed promise in the earlier semi-quantitative-based study (see Table 2 for a synopsis of all of these studies). An important characteristic of this study is its emphasis on measurements in longitudinal CSF samples across time from entry into the ADNI study (BASELINE) to at least 4 years later in order to assess these biomarker trajectories. Such data can be informative to clinical trials that are seeking to use biomarkers as indices of drug engagement and drug effect.
We continue to collaborate with biomarker scientists at UPenn and elsewhere (Irwin, 2017, 2018; Hu, 2010, 2015; Toledo, 2013, 2018; Mattsson, 2013, 2016; Zetterberg and Blennow, 2016). New CSF biomarker-neuropathology correlations done with UPenn collaborators resulted in studies involving CSF tau and histochemical tau in FTLD and CSF tau and A?42 and synucleinopathy in autopsied Lewy Body disorders (Irwin etal, 2017, 2018). Such studies take advantage of the large set of CSF collected at UPenn from individuals prior to death and for whom an autopsy diagnosis provides accurate detection of not only AD neuropathology but concomitant pathologies such as Lewy Bodies, TDP-43 deposits, hippocampal sclerosis. A list of the publications describing the results of these studies thus far is included in a more detailed discussion in “Alzheimer’s Disease Neuroimaging (ADNI) Related Biofluid and Genetic Biomarker Studies Using non-ADNI UPenn Biosamples Followed Longitudinally.”


