Centers and Cores
Industry Scientific Advisory Board
ADNI is governed by a Steering Committee comprised of the PI, all funded Core leaders, all site PIs, representatives of the NIH and FDA, and representatives of the companies contributing funding (observers only). Together with the Executive Committee and the Industry Scientific Advisory Board, these bodies ensure that the ADNI project adheres to the study design and methodology laid out in the grant submission.
Achieving the aims of the ADNI study would not be possible without the collaboration of hundreds of researchers, staff, industry advisors and research volunteers.
Coordination Center
The Coordination Center currently provides data management support for each protocol, including the generation of case report forms, site training, data collection and quality control, study tracking, interim reporting to the Data and Safety Monitoring Board (DSMB), progress reports to the NIH and ongoing daily support to the PI and project directors. Data collection and quality control are managed online. Reported data include: enrollment statistics, demographics, and clinical and cognitive evaluations.
contact
Neuropathology Core

Principal Investigator
John C. Morris, M.D.
Washington University
contact
The Neuropathology Core FRCPath, directed by John C. Morris, M.D. and co-directed by Richard Perrin, MD, Ph.D., follows the following guiding principles:
- Neuropathological examination is essential to validate the clinical diagnoses in the ADNI study groups;
- Variability in methods and interpretation of lesions among individual neuropathologists requires a central laboratory, using state-of-the-art methods and up-to-date criteria to establish uniform and standard neuropathological diagnoses;
- Clinical-neuroimaging-neuropathological correlations in any ADNI participant who comes to autopsy will be of exceptional value; and
- The archiving of fixed and frozen brain tissue will facilitate biomarker studies of the earliest stages of AD.
Dr. John Morris is a member of the Alzheimers Associations Medical & Scientific Advisory Committee. He chairs the Clinical Task Force for the NIAs Alzheimer Disease Centers program. He is author or co-author of over 300 peer-reviewed journal articles and 50 chapters and reviews. He edited the first and second editions of the Handbook of Dementing Illnesses.
Resources
Further Neuropathology Core documents can be found on the Study Documents page.
PET Core

Principal Investigator
William Jagust, M.D.
University of California, Berkeley
contact
The PET Core is responsible for all aspects of PET images including PET acquisition at all performance sites, site qualification, QA and QC of all PET data, tracking all PET data acquisition and processing, and performance of all PET data processing.
Dr. William Jagust has used brain imaging, including PET, to investigate glucose metabolism in patients with AD. His work has included FDG-PET, structural MRI, functional MRI, and most recently amyloid imaging with PET. His laboratory was the first to describe Alzheimer’s-related hippocampal atrophy quantified with MRI, and has continued to pioneer approaches using multimodal imaging to study aging and dementia.
MRI Core

Principal Investigator
Clifford Jack, M.D.
Mayo Clinic, Rochester, Minnesota
contact
The MRI Core is responsible for all aspects of MRI images, including determining specific MRI pulse sequences, site qualification, QA and QC of all MRI data, tracking all MRI data acquisition and processing, and performance of all MRI data processing.
The MRI Core will also utilize an array of publicly available ADNI data, including neuropsychological test results, genotyping, MRI morphometry and other biomarkers to investigate brain-behavior relationships in older adults with and without cognitive impairment. By examining various data, the MRI Core aims to identify patterns of cognitive test performance in groups at increased risk for cognitive decline and investigate the association between these profiles and structural measures of brain volume and cortical thickness.
Resources
Clifford Jack is a highly prominent neuroradiologist in the AD field. His major contributions have been describing changes in hippocampal volume in AD and MCI compared with elderly controls, describing the rates of change in these populations, and correlating these changes with cognition and the transition to MCI and AD.
Further MRI Core documents can be found on the Study Documents page.
Genetics Core

Principal Investigator
Andrew J. Saykin, Psy.D.
Indiana University
contact
The Genetics Core is responsible for preparing genetic data, including for extracting planned and novel MRI and PET endophenotypes for use in whole genome association analyses. In addition to planned analyses (e.g., hippocampal atrophy), the core employs pattern recognition tools to extract novel phenotypic information.
Dr. Saykin joined the Indiana University School of Medicine faculty in November 2006 as director of a new transdisciplinary center of excellence in neuroimaging. His own NIH- and foundation-sponsored research program focuses on the use of brain imaging and genomic methods to study mechanisms of memory dysfunction and treatment response in neurological and psychiatric disorders.
Genetics Core documents can be found on the Study Documents page.
Clinical Core

Principal Investigator
Paul Aisen, M.D.
University of Southern California
contact

Principal Investigator
Ronald Petersen M.D., Ph.D.
Mayo Clinic
contact
The Clinical Core/Coordinating Center for ADNI3, based at the Alzheimers Therapeutic Research Institute (ATRI) at USC, manages the day-to-day clinical operations of ADNI. The Clinical Core oversees ADNI3 clinical activities, contracts with all sites, data management (including creation and management of the ADNI portal in the electronic data capture system for digital upload of all data from clinical sites), tracking and quality control, recruitment and retention of participants, regulatory oversight, financial management of site costs, safety monitoring (including DSMB reporting), and creation of a final “locked dataset” of all data at the close of the study.
Dr. Paul Aisen is a Professor of Neurology and Director of the Alzheimer’s Therapeutic Research Institute at the University of Southern California. The mission of ATRI is to advance the development of new treatment for Alzheimer’s disease (AD) through innovation in clinical trials.
Dr. Ronald Petersen is a Professor of Neurology Director of the Mayo Clinics Alzheimers Disease Research Center and the Mayo Clinic Study of Aging. He has carried out extensive studies in subjects transitioning from cognitively unimpaired to mild cognitive impairment and dementia. He and his colleagues work on biomarkers and cognition in aging.
Resources
Further Clinical Core documents can be found on the Study Documents page.
Biostatistics Core

Principal Investigator
Laurel Beckett, Ph.D.
University of California, Davis
contact
The Biostatistics Core is directed by Dr. Laurel Beckett, Ph.D., at UC Davis (UCD) and consists of biostatisticians based at UCD and USC. The Biostatistics Core provides specific expertise related to the ADNI data, including navigating the database, changes in data acquisition across the phases of ADNI, and working with the data files. This core interacts with biostatisticians and other quantitative researchers from academia and industry who are interested in ADNI data. In addition, the core develops multivariate statistical methods and conducts analyses utilizing data that span the ADNI cores.
Laurel Beckett has been involved in AD research for many years. She was the biostatistician for the East Boston AD studies that first estimated the population prevalence and incidence of AD and projected those numbers to the US population. Her methodological research in longitudinal studies and population-based studies has helped to describe patterns and correlates of clinical decline, both in AD and in the general population.
Resources
References
- Beckett LA, Harvey DJ, Gamst A, Donohue M, Kornak J, Zhang H, Kuo JH; and the Alzheimer’s Disease Neuroimaging Initiative. The Alzheimers Disease Neuroimaging Initiative: Annual change in biomarkers and clinical outcomes. Alzheimer’s and Dementia (2010);6:257-264. PMCID: PMC2867839
- Wyman BT, Harvey DJ, Crawford K, Bernstein MA, Carmichael O, Cole PE, Crane PK, DeCarli C, Fox NC, Gunter JL, Hill D, Killiany RJ, Pachai C, Schwarz AJ, Schuff N, Senjem ML, Suhy J, Thompson PM, Weiner M, Jack CR Jr. Standardization of analysis sets for reporting results from ADNI MRI data. Alzheimer’s and Dementia (2013);9:332-337. PMCID: PMC3891834
- Donohue MC, Sperling RA, Salmon DP, Rentz DM, Raman R, Thomas RG, Weiner M, Aisen PS; Australian Imaging, Biomarkers, and Lifestyle Flagship Study of Ageing; Alzheimer’s Disease Neuroimaging Initiative; Alzheimer’s Disease Cooperative Study. The preclinical Alzheimer cognitive composite; measuring amyloid-related decline. JAMA Neurology (2014);71:961-970. PMCID: PMC4439182
- Beckett LA, Donohue MC, Wang C, Aisen P, Harvey DJ, Saito N; Alzheimer’s Disease Neuroimaging Initiative. The Alzheimers Disease Neuroimaging Initiative phase 2: increasing the length, breadth, and depth of our understanding. Alzheimers & Dementia (2015);11:823- 831. PMCID:PMC4510463
Administrative Core

Principal Investigator
Michael W. Weiner, M.D.
UCSF, NCIRE, VA Medical Center
contact
The Administrative Core oversees all aspects of ADNI, including monitoring all financial and budgetary aspects of the project.
Michael W. Weiner has performed research for over 50 years. He is a Professor of Radiology, Medicine, Psychiatry, and Neurology at the University of California San Francisco (UCSF). He was a Veterans Affairs (VA) Research Associate and VA Clinical investigator and has also worked at VAs in Madison, WI, Palo Alto, CA, and now in San Francisco for the past 37 years where he established the Center for Imaging of Neurodegenerative Diseases (CIND) and is the Director Emeritus. Dr. Weiner was one of the first scientists to perform NMR on an intact animal and has used MRI/MRS for clinical research since 1984. Since 1988, he has focused on neurodegenerative diseases, particularly Alzheimer’s disease. Dr. Weiner has published over 900 peer-reviewed papers.
He is the founder of and PI of the Alzheimer’s Disease Neuroimaging Initiative (ADNI), three Department of Defense ADNI grants (concerning the relationship of PTSD and TBI to AD), among other projects and is the founder of the Brain Health Registry. Dr. Weiner is a participant in ADNI at UCSF and has undergone all ADNI procedures (including lumbar punctures) longitudinally since the onset of the project 13 years ago. He oversees all aspects of the ADNI project and directs each stage of the investigation. He works closely with the core leaders, the various members of the scientific team, and the NIA Program Administrator. Dr. Weiner leads the ADNI Executive Committee teleconferences, attends the ADNI Clinical Core teleconferences, and often joins other ADNI Core meetings. He leads the ADNI Steering Committee meeting and attends all Scientific Advisory Board Meetings and is responsible for lending direction to all projects within this Initiative, based on input received by the Private Partner Scientific Board (PPSB), the Scientific Advisory Board (SAB) and the Resource Allocation Review Committee (RARC). He is responsible for NIA Progress Reports, non-competitive renewals, and competitive renewals. Dr. Weiner responds to many questions and inquiries concerning ADNI and he is passionate concerning the open data sharing of all ADNI data without embargo. Finally, Dr. Weiner is closely involved in writing abstracts and manuscripts and lectures at scientific meetings around the world concerning the progress and success of ADNI.
Resources
Informatics Core

Principal Investigator
Arthur W. Toga, Ph.D.
Laboratory of Neuro Imaging
University of Southern California
contact
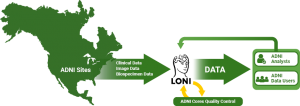
The Informatics Core, based at the Laboratory of Neuro Imaging (LONI) at the University of Southern California, is responsible for de-identifying, archiving, and disseminating all clinical, biospecimen, genetic and imaging data including raw and processed MR and PET scans. All data are made available to approved ADNI investigators within days after the date of collection through the Image and Data Archive (IDA), which provides data search, exploration and download interfaces for evaluating and obtaining data of interest. The Informatics Core also distributes methods and software tools created and/or used by the ADNI analysts. The Informatics Core provides data access and support to thousands of investigators from more than 100 countries who are using ADNI data in their research.
Arthur W. Toga is a Professor of Neurology at USC, director of the Mark and Mary Stevens Neuroimaging and Informatics Institute, founder and director of the Laboratory of Neuro Imaging (LONI), and the founding editor of the journal NeuroImage. Dr. Toga has career-long funding and distinguished publication record in the analysis, registration, and modeling of structural and functional images obtained from many species, including humans.
Resources
Further Informatics Core documents can be found on the Study Documents page.
Biomarker Core

Principal Investigator
Leslie Shaw, Ph.D.
University of Pennsylvania
contact
The goals of the Biomarker Core, directed by John Trojanowski, M.D. and co-directed by Leslie Shaw, Ph.D. at the University of Pennsylvania, are to establish a bank of biological fluids from the unique cohort of subjects followed in ADNI and conduct studies of selected AD biomarkers:
- CSF A?1-42, t-tau and p-tau181 for all ADNI1/GO/2 CSFs; A A?1-40 added to this analyte group for all CSFs collected in ADNI3.
- These CSF analyses are done in partnership with Roche using Elecsys immunoassays on the fully automated Cobas e e601 analyzer
- CSF A?1-42/1-40/1-38 analyses of all ADNI1/GO/2 CSFs by validated UPLC/tandem mass spectrometry
- Analyses of plasma biomarkers in partnership with Roche is anticipated on the fully automated Cobas e601
- A wide array of new candidate biomarkers analyzed by RARC-approved investigators in ADNI CSF, plasma or serum samples using novel immunoassays or HPLC/tandem mass spectrometry.
- Studies in non-ADNI UPenn and collaborator AD and non-AD NGD with neuropathologic diagnoses that are of key interest to Biomarker Core PIs and relate to ADNI studies involving mixed pathology in AD subjects.
- In support of harmonization of A?1-42 measurements we have participated in the development of mass spectrometry-based reference methodology and mass assignment of concentration values to the newly released Certified Reference Material for this key AD biomarker; in addition we are participating in studies of pre-analytical factors affecting CSF A?1-42 concentration and strategies to minimize these sources of variability.
References
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Figurski M, Coart E, Blennow K, Soares H, Simon AJ, Lewczuk P, Dean RA, Siemers E, Potter W, Lee VM, Trojanowski JQ, Initiative AsDN. Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta Neuropathol 2011;121:597-609.
- Hansson O, Seibyl J, Stomrud E, Zetterberg H, Trojanowski JQ, Bittner T, Lifke V, Corradini V, Eichenlaub U, Batrla R, Buck K, Zink K, Rabe C, Blennow K, Shaw LM, for the Swedish BioFINDER study group and the Alzheimer’s Disease Neuroimaging Initiative. CSF biomarkers of Alzheimer’s disease concord with amyloid-? PET and predict clinical progression: A study of fully-automated immunoassays in BioFINDER and ADNI cohorts. Alz Dement 2018; https://doi.org/10.1016/j.jalz.2018.01.010.
- Korecka M, Waligorska T, Figurski M, Toledo JB, Arnold SE, Grossman M, Trojanowski JQ, Shaw LM. Qualification of a surrogate matrix-based absolute quantification method for amyloid-beta42 in human cerebrospinal fluid using 2D UPLC-tandem mass spectrometry. J Alzheimers Dis 2014;41:441-51.
- Shi M, Tang L, Toleddo JB, Ginghina C, Wang H, Aro P, Jensen PH, Weintraub D, Chen-Plotkin AS, Irwin DJ, Grossman M, McCluskey L, Elman LB, Wolk DA, Lee EB, Shaw LM, Trojanowski JQ, Zhang J. Cerebrospinal fluid ?-synuclein contributes to the differential diagnosis of Alzheimers disease. Alz Dement 2018, in press: https://doi.org/10.1016/j.jalz.2018.02.015
- Irwin DJ, Xie SX, Coughlin D, Nevler N, Akhtar RS, McMillan CT, Lee EB, Wolk DA, Weintraub D, Chen-Plotkin A, Duda JE, Spindler M, Siderowf, Hurtig HI, Shaw LM, Grossman M, Trojanowski JQ. CSF tau and amyloid-? predict cerebral synucleinopathy in autopsied Lewy body disorders. Neurology 2018; doi:10.1212/WNL.000000000000516.
- Kuhlmann J, Andreasson U, Pannee J, Bjerke M, Portelius E, Leinenback A, Bittner T, Korecka M, Jenkins RG, Vanderstichele H, Stoops E, Lewczuk P, Shaw LM, Zegers I, Schimmel H, Zetterberg H, Blennow K. on behalf of the IFCC Working Group on Standardization of CSF proteins (WG-CSF). CSF A?1-42 “an excellent but complicated Alzheimer’s biomarker” a route to standardization. Clin Chim Acta 2017; 467:27-33.
John Q. Trojanowski obtained his MD/PhD in 1976 from Tufts University, completed his internal medicine internship at Mt. Auburn Hospital, and completed his pathology and neuropathology at Massachusetts General Hospital and the University of Pennsylvania Perelman School of Medicine, where he joined the faculty in 1981. He is Professor of Pathology and Laboratory Medicine, Director of the NIA Alzheimers Disease Center, the NINDS Morris K. Udall Parkinsons Disease Center, and the Institute on Aging. His research focuses on Alzheimer’s (AD) and Parkinson’s (PD) disease, amyotrophic lateral sclerosis (ALS), frontotemporal degeneration (FTD) which led to the discovery of the major disease proteins in these disorders, i.e. tau, alpha-synuclein and TDP-43 proteins, and that aggregation of these pathological proteins is a common mechanism underlying these disorders thereby opening new avenues for drug discovery to treat these disorders. Dr. Trojanowski is among the top 10 most highly cited AD researchers from 1997 to 2007 with an h-index of 203.
Leslie M Shaw, PhD, directs the Biomarker Research Laboratory at the Perelman School of Medicine, University of Pennsylvania, in the Department of Pathology and Laboratory Medicine. He is PI and co-director of the ADNI Biomarker Core laboratory and co-leads the Biomarker Core of the UPenn ADCC. He has published more than 280 scientific papers and reviews in peer-reviewed literature. Amongst the major interests of Dr Shaw are the development and validation of methods for quantification of CSF AD biomarkers including A? 42 and related A? peptides, t-tau and p-tau181 and new promising biomarkers in CSF and plasma for early disease detection, their predictive performance for AD disease progression and relationships to imaging biomarkers in AD and AD-related disorders.
Resources



















